
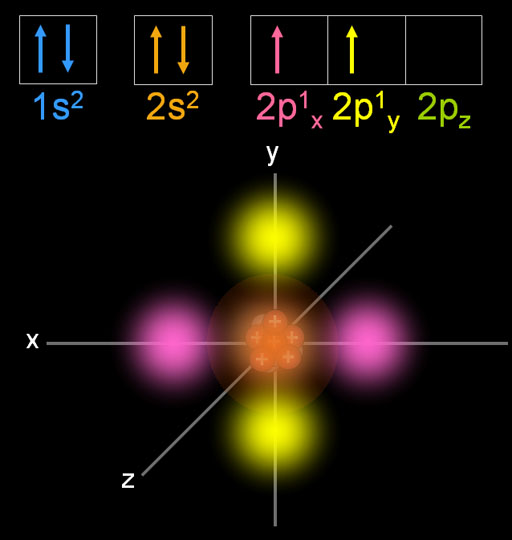
However, diamond is an excellent heat conductor. It is a poor conductor, because all electrons are localized in the chemical bonds. It is the hardest stone, much harder than anything else in the material world. The bonding has given diamond some very unusual properties. The bond length of 154 pm is the same as the C-C bond length in ethane, propane and other alkanes.Īn idealized single crystal of diamond is a gigantic molecule, because all the atoms are inter-bonded. The bonding, no doubt, is due to the sp 3 hybrid orbitals. In the crystal, every carbon atom is bonded to four other carbon atoms, and the bonds are arranged in a tetrahedral fashion. The length of the carbon-hydrogen bonds in methane is 109 pm.ĭiamond is a crystal form of elemental carbon, and the structure is particularly interesting. This is simply a restatement of the Valence Shell Electron Pair Repulsion (VSEPR) theory that you learned in General Chemistry: electron pairs (in orbitals) will arrange themselves in such a way as to remain as far apart as possible, due to negative-negative electrostatic repulsion.Įach C-H bond in methane, then, can be described as a sigma bond formed by overlap between a half-filled 1 s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp 3 hybrid orbitals in the central carbon. This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible. ( select ‘load sp 3‘ and ‘load H 1s’ to see orbitals). In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp 3 orbital. In this picture, the four valence orbitals of the carbon (one 2 s and three 2 p orbitals) combine mathematically (remember: orbitals are described by wave equations) to form four equivalent hybrid orbitals, which are called sp 3 orbitals because they are formed from mixing one s and three p orbitals. In order to explain this observation, valence bond theory relies on a concept called orbital hybridization. How does the carbon form four bonds if it has only two half-filled p orbitals available for bonding? A hint comes from the experimental observation that the four C-H bonds in methane are arranged with tetrahedral geometry about the central carbon, and that each bond has the same length and strength.
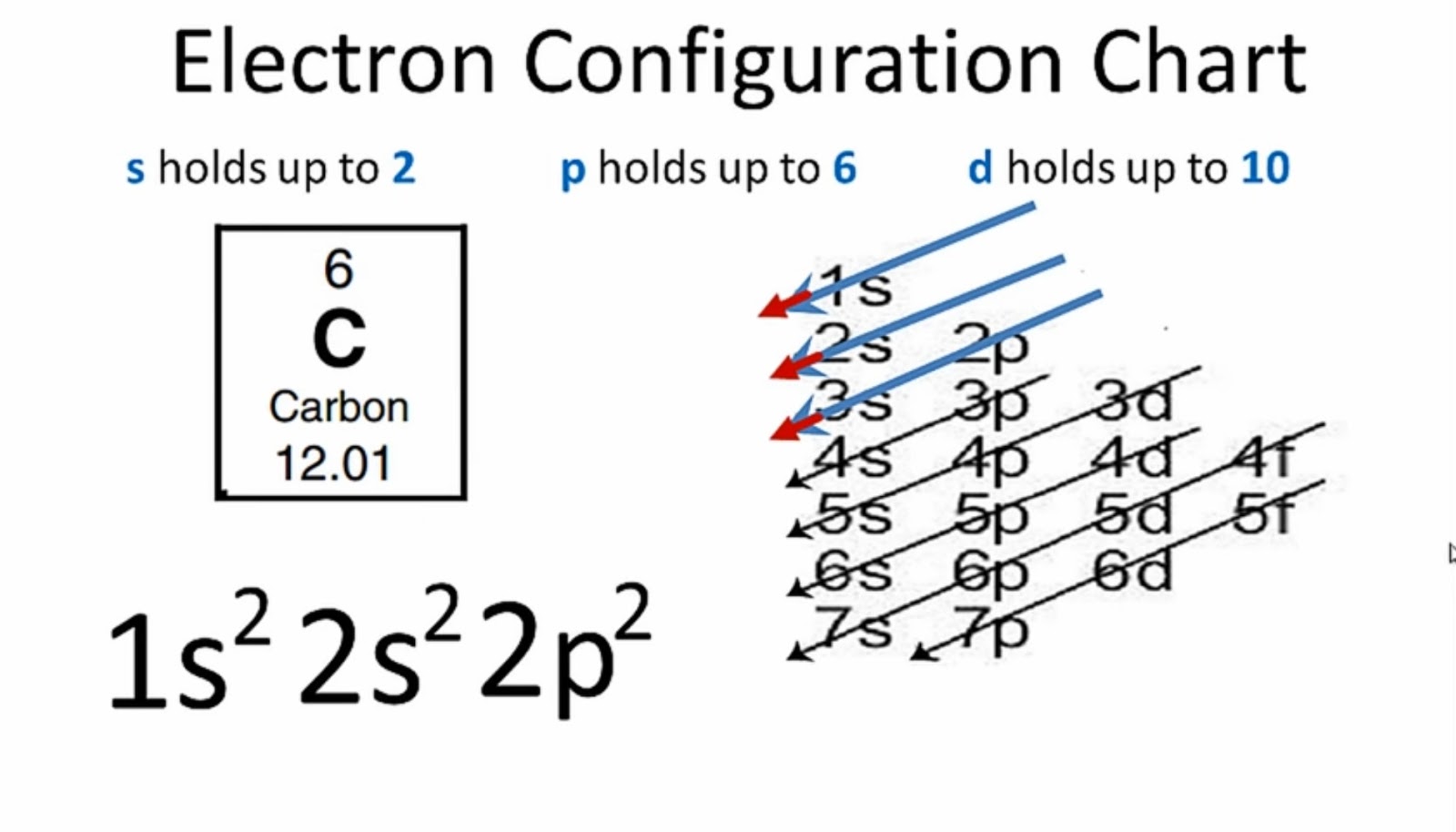
This picture is problematic when it comes to describing the bonding in methane.


 0 kommentar(er)
0 kommentar(er)
